Chemical biocides have been utilized in cooling towers to prevent biological growth for multiple decades. There are many commercially available chemical biocides, including liquid bleach, activated bromine products, and stabilized chlorine and/or bromine products. Stabilized bromine is a popular choice as a biocide due to its enhanced product stability, efficacy in high pH ranges, and its relatively safe means of chemical handling and pumping compared to other biocide products.
Historically, stabilized bromine concentration has been measured using oxidation-reduction potential (ORP) sensors. ORP sensors provide a measurement of the amount of electrochemically active species present in a sample but are unable to distinguish between chemical species. Given that cooling towers require several chemical additives to operate within compliance, an ORP measurement does not yield the most accurate insight into the stabilized bromine concentration in the tower.
A better alternative to ORP sensors for this application is the use of “bare” or “open cell” amperometric sensor technology. Bare amperometric sensors have shown repeated success in measuring and dosing free and combined chlorine products as well as activated bromine products. Due to their solid-state design, bare amperometric sensors provide an easily interpretable signal that directly relates to the concentration of chlorine and bromine species present.
To demonstrate that bare amperometric sensors could accurately measure stabilized bromine products, we installed a sensor in a 200-ton cooling tower at an industrial plant in the suburbs of Chicago. The cooling tower for this case study is timer-fed, meaning a highly concentrated solution of biocide is introduced at the beginning of the tower’s operation cycle and is consumed as the tower runs. For the first part of the case study, we measured the signals from a standard ORP sensor and the bare amperometric sensor during the tower’s normal operation. We performed standard DPD reference measurements to show that the bare amperometric sensor accurately measured the halogenated biocide concentration.
For the second phase of the study, we established control using a chemical dosing pump that is directly informed by the bare amperometric sensor signal. This residual-based control uses less biocide compared to timer-feeding since biocide is added only when the concentration determined by the sensor approaches the lower set limit of the biocide program. This provides greater process control and greatly reduces chemical costs, in addition to preventing corrosion of the cooling tower caused by high biocide concentrations. Through this case study, we hope to demonstrate the viability and utility of bare amperometric sensors for measuring stabilized bromine concentration in industrial cooling towers.
Cooling towers have been used to exchange heat via evaporation in buildings and in industrial processes since the 19th century.1 Applications which utilize cooling towers to manage waste heat generation include thermoelectric power generation, petroleum refineries, natural gas processing plants, food processing plants, and many other industrial processes.2
Cooling towers work by exchanging heat between hot air and ambient temperature water. When the cool water contacts the hot air, heat transfer occurs, and some of the water evaporates. The evaporated water becomes a fine mist which flows out of the top of the tower, carrying some of the heat with it. The water which does not evaporate is collected at the bottom of the tower.3
The conditions under which cooling towers operate make them an ideal environment for microbiological growth. One pathogen of concern is Legionella bacteria, which can lead to Legionnaires’ disease if inhaled. Managing growth of Legionella and other pathogens in cooling towers is a major health concern, and regulations centered around this practice come mainly from the Center for Disease Control (CDC).4
According to the CDC, the best way to control Legionella growth is by installing an automated water treatment system.4 Cooling towers are treated with chemical biocides, which kill pathogens that might be present. Most often, oxidizing chemical biocides such as chlorine- or bromine-containing reagents are used, and there should always be a measurable residual of chemical biocide during operation of the cooling tower. To remain within compliance, the chemical biocide residual should be continuously monitored and recorded.
The practice of adding chlorinated chemical biocides to water for the purpose of disinfection began in the 19th century.1 Early treatments combined chlorine gas (Cl2) and calcium hydroxide (Ca(OH)2) to form chlorinated lime, or calcium hypochlorite (Ca(OCl)2). Other treatments utilized liquified chlorine gas, liquid bleach (NaOCl), or chloramines (R-NHCl).
Though the chlorinated chemical biocide products used vary in form, cost, and ease of handling, they all produce hypochlorous acid (HOCl) when added to water:
1) Cl2(g) + H2O(l) ↔ HOCl(aq) + HCl(aq)
2) NaOCl(aq) + H2O(l) ↔ HOCl(aq) + NaOH(aq)
3) Ca(OCl)2(s) + 2H2O(l) ↔ 2HOCl(aq) + Ca(OH)2(aq)
Hypochlorous acid further dissociates into the hypochlorite ion (OCl– ) and a proton (H+), as shown by Equation 4. Figure 1 shows this dissociation as a function of pH. As the pH increases, the relative amount of hypochlorous acid decreases, and the amount of hypochlorite ion increases. At pH 6, the sample is 97% HOCl and 3% OCl– . By pH 9, the sample is only 3% HOCl and 97% OCl– .
4) HOCl(aq) ↔ H+(aq) + + OCl–(aq)
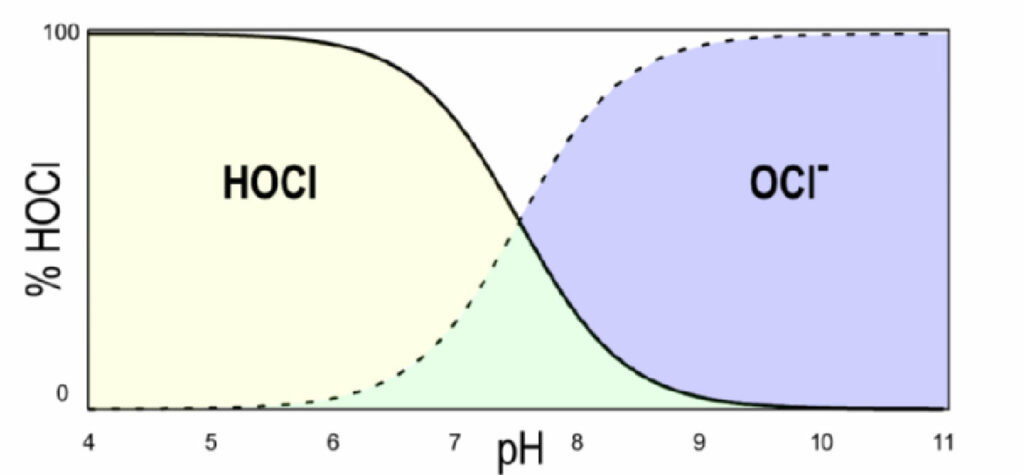
Figure 1. Dissociation curve of hypochlorous acid (HOCl) as a function of pH.
While both hypochlorous acid and hypochlorite ion possess disinfection capability against waterborne pathogens, hypochlorous acid is about 10 times more effective.5 While this process is not completely understood, it is thought that HOCl disrupts the cell membrane of a pathogen due to its relatively small size, neutral charge, and highly oxidizing nature.6 The upper pH limit of an application utilizing only hypochlorous acid is around 8.5, since the relative amount of available hypochlorous acid is very small above this pH.
There are several commercial products available for dosing hypochlorous acid into water. Liquid bleach and solid calcium hypochlorite products are commonly used due to their relatively safe and easy means of handling. Both bleach and calcium hypochlorite are considered “activated” products, meaning that they readily produce free chlorine immediately in water. 7 The term “free chlorine” refers to the total concentration of hypochlorous acid, hypochlorite ion, and molecular chlorine.
There are also many commercially-available “stabilized” chlorine biocide products used in industrial cooling towers. Stabilized chlorine products utilize “combined chlorine” – a chlorine atom bound to a stabilizer, typically amine groups or a larger organic molecule such as hydantoins or sulfamates.7 Examples of common combined chlorine species are shown in Figure 2.
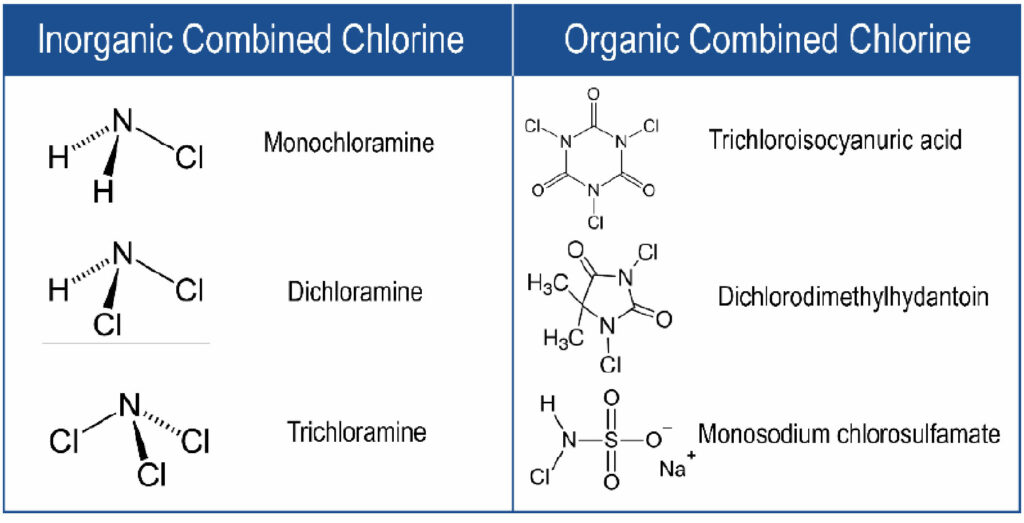
Figure 2. Structures of common combined chlorine species, including inorganic combined chlorine species (left) and organic combined chlorine species (right).
Stabilized chlorine products also yield hypochlorous acid in water, but at much slower rates. The decreased rate of release of stabilized products compared to activated products is due to differences in chemical composition. The presence of the stabilizer increases efficacy in high-demand applications such as cooling towers while also increasing shelf life.7
When discussing activated and stabilized chlorine products, it is important to distinguish between free and total chlorine. As stated above, free chlorine is defined as the total concentration of hypochlorous acid, hypochlorite ion, and molecular chlorine present in a sample (Equation 5). Combined chlorine refers to chlorine bound to a stabilizer, such as monochloramine (NH2Cl) or organic chlorine species (RCln, R = organic molecule, n = number of chlorine atoms), shown by Equation 6. Total chlorine is defined as the sum of all free chlorine and combined chlorine present (Equation 7).
5) Free chlorine = Cl2 + HOCl + OCl–
6) Combined chlorine = NH2Cl + NHCl2 + NCl3 + RClx
7) Total chlorine = Free chlorine + Combined chlorine
Total chlorine = Cl2 + HOCl + OCl– + NH2Cl + NHCl2 + NCl3 + RCln
Distinguishing between free and total chlorine is important for two reasons: first, compliance regulations usually require a specific metric to be reported (either free chlorine OR total chlorine). Second, there are differences in the measurement techniques used to quantify free chlorine and total chlorine concentrations, which will be discussed in later sections.
In addition to activated and stabilized chlorine products, there are several brominecontaining biocide products available. Chlorinated and brominated biocides can generally be referred to as “halogenated biocides” since they contain halogen atoms (from Group 7 on the Periodic Table, including chlorine, bromine, and iodine). Like chlorine products, bromine products yield hypobromous acid (HOBr) in water. Hypobromous acid further dissociates into a proton and the hypobromite ion (OBr– ):
5) HOBr(aq) ↔ H+(aq) + OBr– (aq)
The dissociation of hypobromous acid exhibits similar pH-dependence compared to hypochlorous acid, as shown by Figure 3. One distinct advantage of using brominecontaining biocide products is their enhanced efficacy at higher pH compared to chlorine-containing biocide products. The addition of hypobromous acid expands the upper pH limit of an application to around 9.5, compared to the upper limit of 8.5 for applications using only hypochlorous acid.7
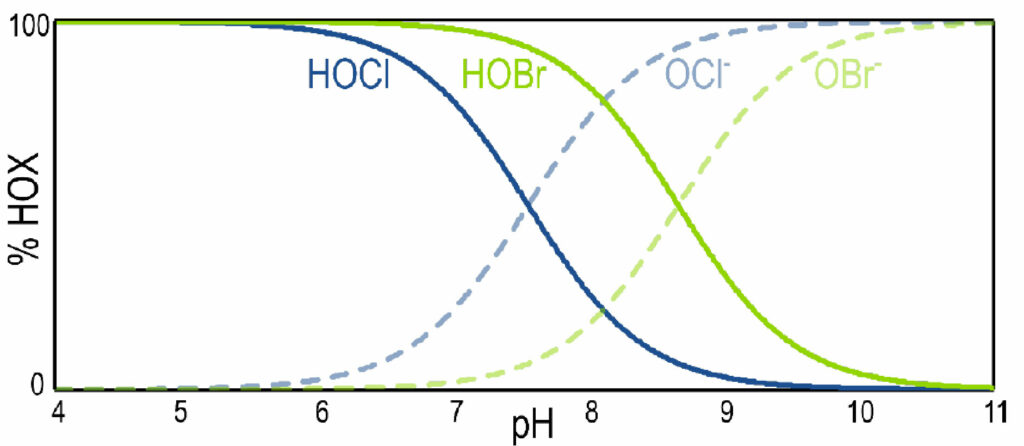
Figure 3. Theoretical dissociation curves of HOCl/OCl– (solid blue line/dashed blue line) and HOBr/OBr– (solid green line/dashed green line) as a function of pH.
Bromine-containing biocide products are available in both activated and stabilized forms. Both are typically used in addition to chlorine-containing biocide products in well-defined ratios. Stabilized bromine products are of particular interest due to the improved corrosion control afforded by their higher upper pH limit of 9.5.7 Stabilized bromine products are easier to handle and store compared to pure bleach or activated bromine products, making them an attractive choice for cooling tower applications.
Scientists have been using colorimetric techniques to quantify concentration since the 19th century.8 Colorimetric techniques use chemical reactions which result in a color change to measure concentration. Typically, a reagent is added to a sample, where it reacts with the species of interest, or the analyte. The reaction between the reagent and the analyte causes a color change (either from colorless to colored or colored to colorless). The intensity of the color change is measured by a colorimeter, which shines light of a specific wavelength at the sample and detects how much light the sample absorbs. By tuning the wavelength of light and the reagent used to react with the analyte, colorimetric techniques can achieve accurate and reliable concentration measurements.
Both chlorine- and bromine-containing biocides are commonly measured using colorimetric techniques with N,N′-diethyl-p- phenylene-diamine (DPD) reagents. When DPD is added to a sample containing chlorine or bromine biocides, the DPD reagent is oxidized, resulting in a color change from a colorless to pink. The intensity of the pink color developed can be measured and directly correlated to a halogenated biocide concentration. When a mixture of chlorine- and bromine-containing biocides is used, a DPD measurement reflects the total amount of halogenated biocide present and does not distinguish between chlorine and bromine species. A free halogenated biocide measurement uses DPD-1 reagent, while a total halogenated biocide measurement uses DPD-4. DPD-4 reagent includes potassium iodide (KI) to convert combined halogen species to free halogen species, since combined halogen species do not readily oxidize DPD on their own.
Colorimetric techniques have distinct disadvantages in cooling tower applications. Because the measurement is an optical measurement (meaning light must pass through the sample), samples must be optically clear (i.e., free of solids and suspended particles). Cooling tower samples often have high amounts of solids present, which disrupt colorimetric measurements and provide false positive residual readings. Colorimetric techniques are also incompatible with applications that require continuous monitoring since the minimum time between samples in an on-line colorimetric analyzer is 2.5 minutes. Since cooling towers must be monitored and controlled at all times during operation, an on-line analyzer with a continuous measurement is needed.
Because of the limitations of colorimetric techniques, electrochemical techniques are of interest for measuring chemical biocides. Electrochemical techniques correlate a measurable electrical signal (potential or current) to a chemical property (e.g. concentration) of a sample. Often, electrochemical techniques are used to measure the nature of oxidation-reduction (redox) reactions, such as the reactions that take place between chemical biocides and waterborne pathogens.
The distinct advantage of electrochemical measurements is that they offer continuous measurements with narrow time resolution. Electrochemical techniques are used to measure a wide variety of processes, since they have the capability to be highly tunable and selective for a particular analyte. The selectivity of an electrochemical technique depends mostly upon the construction materials of the sensor.
One of the first electrochemical techniques used to measure chemical biocide concentration is the oxidation-reduction potential (ORP) measurement. An ORP measurement quantifies the flow of electrons between an oxidant (e.g. chemical biocide) and a reductant (e.g. waterborne pathogens). As the concentration of oxidant increases, the ORP of the system increases. Since many chemical biocides disinfect via redox reactions, the ORP value of a system can be roughly correlated to the chemical biocide concentration present.
An ORP measurement cannot distinguish between chemical species in a system. Any species that is electrochemically “active” – i.e. it possesses the ability to either raise or lower the ORP value via oxidation or reduction – will affect an ORP measurement. One common electrochemically active species found in cooling towers is sulfuric acid, which is used to lower the pH of the makeup water within the tower. An acid feed will cause the ORP signal to increase, causing false positive biocide residual signals. Another species which can cause false positive readings on an ORP sensor is dissolved oxygen, which increases in concentration as the temperature decreases.
ORP sensors usually respond quickly to an increase in ORP signal (e.g., after a biocide feed), but are slow to react as the biocide is consumed. In other words, the actual biocide concentration decreases at a faster rate than the ORP signal decreases. Because of this “lag,” systems which use the ORP signal to automatically dose biocide are at risk for operating under conditions in which it appears that a biocide residual is present, but the actual biocide concentration is below the minimum allowed limit. With compliance regulations becoming increasingly strict, cooling tower operators require constant validation and assurance of their biocide residual, which ORP sensors cannot reliably provide.
Another electrochemical technique used to measure chemical biocides is amperometry. A technique which is “amperometric” is one in which an electrical current is measured. An amperometric sensor can be thought of as an electrochemical cell, consisting of electronic conductors (“electrodes”) and ionic conductors (“electrolytes”).9
For amperometric biocide measurements, the current produced from a specific electrochemical reaction is used to calculate the concentration of the analyte of interest. A typical amperometric sensor consists of a measuring electrode and a counter electrode, which are often made from precious metals such as gold or platinum. Many modern amperometric sensors also utilize a reference electrode, typically a silver/silver chloride electrode. Each electrode has a defined purpose and is designed to maximize specificity for the analyte of interest.
The purpose of the counter electrode is to allow current to flow. The electrochemical system must be electrically balanced, and the counter electrode passes the current needed to balance the current produced at the working electrode.
The reference electrode is made from a material with a well-known oxidation/reduction potential and provides a highly stable “starting point” from which all potentials in the system are calculated. The oxidation/reduction reactions that happen at the reference electrode are the same regardless of the target analyte.
For halogenated biocide measurements, the electrochemical reaction of interest is:
5) HOX + H+ + 2e− ↔ X− + H2O
where X is either chlorine (Cl) or bromine (Br). The current yielded by the reduction of either hypochlorous acid (HOCl) or hypobromous acid (HOBr) is translated to a halogenated biocide concentration. When combined halogen species are present, a catalytic electrolyte is necessary since combined halogen species each have different reduction potentials and may not be reduced at the applied potential of the measuring electrode.
The focus of this study is to use bare amperometric sensors to measure stabilized bromine biocide residuals. A bare amperometric sensor consists of the electrodes described above (measuring, counter, and reference electrode), which are in direct contact with the water from the cooling tower. Bare amperometric sensors polarize quickly and are ready to measure within an hour of installation (compared to membrane-covered sensors, which can take 8-24 hours to commission). The sensors are installed into flow-regulated sample cell, and the effects of flow and temperature fluctuations are engineered out of the equation. Thus, the bare amperometric sensors chosen for this study yield a highly-interpretable signal which directly measures stabilized bromine biocide residuals.
The cooling tower used for this study is a 200-ton cooling tower at an industrial plant outside of Chicago, IL. Municipal water sourced from Lake Michigan is used as the cooling tower’s makeup water. Before this study, the cooling tower was timer-fed with a stabilized bromine chemical biocide one or two times per day. The chemical biocide used is Brommax®, a single-drum bromine biocide stabilized with sulfamic acid. The average amount of water processed by the cooling tower is about 5000 gallons per day, with an average discharge of about 700 gallons per day. The cooling tower previously used an ORP measurement to show biocide was dosed after a timer-feed.
To investigate the efficacy of a bare amperometric sensor for measuring and controlling a stabilized bromine residual, we installed a Kuntze Krypton® DIS with a Zirkon® DIS Total sensor onto a sample line pulled from the cooling tower. Photos of the analyzer and the cooling tower are shown in Figure 4.
The Zirkon® DIS Total sensor is a bare amperometric sensor with a platinum microparticle film working electrode and a platinum ring counter electrode, shown in Figure 5. The sensor is filled with a catalytic electrolyte which enables it to measure combined halogen species in addition to free halogen. This sensor is marketed as a total chlorine sensor and is typically used in applications that utilize monochloramine or other

Figure 4. Photos of the Kuntze Krypton® DIS (left) and the 200-ton cooling tower (right) used for the study.
stabilized chlorine products. To our knowledge, this study is the first application of a bare amperometric sensor for monitoring stabilized bromine residuals.
It is important to note that the Zirkon® DIS Total sensor measures the total concentration of halogenated biocide, which is a combination of chlorine- and brominecontaining species. It cannot distinguish between species (e.g. between HOCl and HOBr), and the raw measurement signal depends strongly upon the type of biocide used. Different ratios of bromine, chlorine, and stabilizer will produce different signal strengths. The benefit of the direct biocide measurement enabled by a bare amperometric sensor is an easily interpretable measurement signal, which can be directly related to biocide concentration (unlike an ORP measurement signal).

Figure 5. Photograph of the Kuntze Zirkon® DIS Total sensor.
First, we established the sensor’s accuracy by comparing the sensor readings to DPD reference measurements. During this phase of the study, the biocide dosing of the cooling tower was still under the control of the ORP sensor. Once confidence in the sensor’s capability was established, the Krypton® DIS system was put into direct control of the dosing pumps. The target biocide residual was between 1.5 to 2.0 parts per million (ppm).
We first installed the Krypton® DIS system and monitored the stabilized bromine concentration while comparing to DPD-4 reference measurements. A small portion of this test is shown in Figure 6. We found that the bare amperometric sensor’s measurements were reproducibly within ± 15% of the DPD reference measurements.
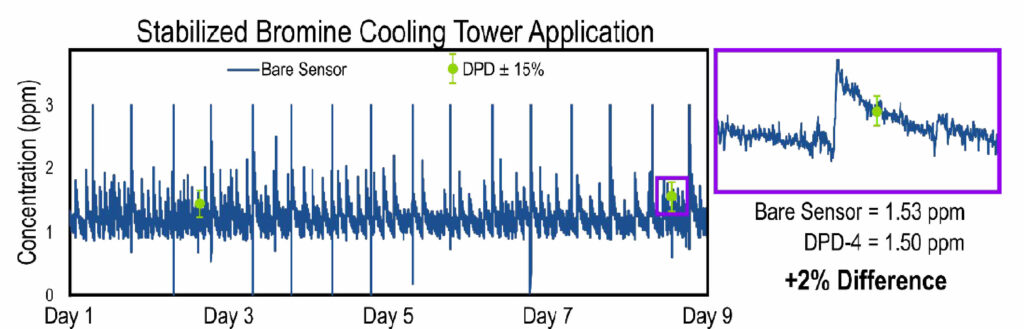
Figure 6. Plot establishing sensor accuracy for measuring stabilized bromine residuals. The bare amperometric sensor signal (dark blue line) matches well with the DPD reference measurements (green circles). The error bars on the DPD measurements represent a ± 15% tolerance with respect to the DPD measurement. The purple box shows a zoomed-in view to highlight the bare sensor’s accuracy with respect to the DPD measurement.
After establishing the sensor’s capability for accurately measuring stabilized bromine residuals, we placed the cooling tower’s dosing pumps under direct control of the Krypton® DIS system. The Krypton® DIS was wired directly to the dosing pump via a drycontact relay. The target stabilized bromine biocide residual was between 1.5 to 2.0 ppm. Once the cooling tower was controlled via the Krypton® DIS, the biocide residual stayed between 1.0 and 3.0 ppm, centered around the setpoint. The results of this phase of the study are shown in Figure 7.
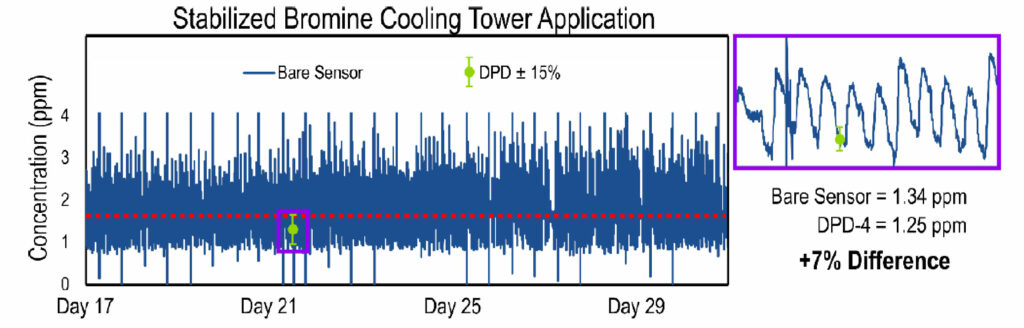
Figure 7. Plot establishing the Krypton® DIS’s ability to control to setpoint. The bare amperometric sensor signal (dark blue line) varies between 1.0 and 3.0 ppm, and is centered around the target setpoint (red dashed line). The sensor signal matches well with the DPD reference measurements (green circles). The error bars on the DPD measurements represent a ± 15% tolerance with respect to the DPD measurement. The purple box shows a zoomed-in view to highlight the bare sensor’s accuracy with respect to the DPD measurement.
We found that the Kuntze Zirkon® DIS Total bare amperometric sensor is capable of accurately measuring and controlling a stabilized bromine residual in an industrial cooling tower. The cooling tower was previously timer-fed one to two times per day, which did not provide the tower operators with “peace of mind” that microbiological growth was being managed. After the installation of the Krypton® DIS system to measure and control biocide residual, the tower operator reported feeling much more confident in their water treatment program, since they felt confident that there was a constant biocide residual in the cooling tower.
The future direction of this study is to decrease the target setpoint of the biocide residual. This will decrease the amount of biocide discharged during tower blowdowns as well as save on chemical costs. The confidence afforded by the direct biocide concentration from the bare amperometric sensor signal is what makes this step possible and logical.
(1) Race, J. Chlorination of Water; John Wiley and Sons, Inc., 1918.
(2) Centers for Disease Control and Prevention. Other Uses and Types of Water https://www.cdc.gov/healthywater/other/industrial/cooling_towers.html (accessed 2021 -06 -01).
(3) U.S. Geological Survey. National Handbook of Recommended Methods for Water Data Acquisition https://pubs.usgs.gov/chapter11/chapter11J.html (accessed 2021 – 05 -24).
(4) Centers for Disease Control and Prevention. Controlling Legionella in Cooling Towers https://www.cdc.gov/legionella/wmp/control-toolkit/cooling-towers.html (accessed 2021 -06 -01).
(5) National Research Council (US) Safe Drinking Water Committee. The Disinfection of Drinking Water; National Academies Press (US), 1980.
(6) Calomiris, J. J.; Christman, K. A. How Does Chlorine Added to Drinking Water Kill Bacteria and Other Harmful Organisms? Why Doesn’t It Harm Us? Sci. Am. 1998, 278 (5).
(7) Bajpai, P. The Control of Microbiological Problems. Pulp Pap. Ind. 2015, 103–195. https://doi.org/10.1016/B978-0-12-803409-5.00008-2.
(8) Rinsler, M. G. Spectroscopy, Colorimetry, and Biological Chemistry in the Nineteenth Century. J. Clin. Pathol. 1981, 34 (3), 287–291. https://doi.org/10.1136/jcp.34.3.287.
(9) Bard, A. J.; Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons, Inc., 2000.